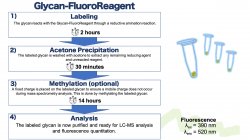
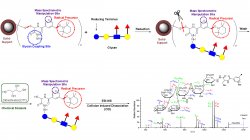
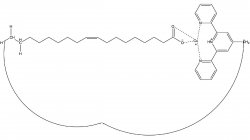
The Gao lab investigates the characterization, discrimination, and quantitation of glycans, lipids, peptides, glycolipids, and glycopeptides via liquid chromatography-mass spectrometry (LC-MS). By combining tools of organic chemistry, biochemistry, mass spectrometry, and free-radical chemistry, the Gao lab has developed reagents and a suite of new technologies. Recently, the synthesis of a novel reagent with applications of free-radical chemistry and fluorescence quantitation for glycans, glycopeptides, and glycolipids is currently seeking the patenting and commercialization steps towards future use by labs around the world.
Jump to:
Research
The Gao lab is interested in studying the discrimination, characterization, and quantitation of biomolecules by integrating the tools of free-radical chemistry and liquid chromatography-mass spectrometry. A summary of recent work in the Gao lab is described below:
People

Professor, Chemistry and Biochemistry
- Phone
- 973-655-5136
- gaoj@montclair.edu
- Location
- Richardson Hall, 344
Staff

Rayan Murtada, Research Assistant/Lab Manager
Rayan earned his BS in Biochemistry at Montclair State University. With an interest in fluorescence quantitation, organic chemistry, and biochemistry, he is interested in the development and synthesis of novel tags while expanding their applications to a breadth of biomolecules (e.g., glycans, lipids, and peptides, and so forth) for rapid and accurate analyses via liquid chromatography-mass spectrometry (LC-MS) and fluorimetry. Rayan also focuses his effort towards the discovery of bioconjugate fragmentation mechanisms. Outside of the lab, Rayan is an avid hiking enthusiast and can sometimes be found flying thousands of feet above the ground in pursuit of earning his private pilot license.

Shane Finn
Shane is working towards earning his B.S. in Chemistry from Montclair State University. His interests lie in analytical chemistry, novel reagent synthesis, and liquid chromatography coupled with mass spectrometry. Shane specializes in the further analysis of purified bioconjugates via liquid chromatography-mass spectrometry (LC-MS) and synthetic chemistry of multi-functional reagents. He seeks to pursue future involvement in postgraduate research and attain a Ph.D. in analytical chemistry. Outside of research, Shane enjoys exploring different hike trails and the scenic routes of the east coast states.

Ray Stanton
Ray is a senior undergraduate student who will be graduating with a BS in Chemistry in Spring 2022. In Dr. Gao’s lab, Ray assists in the development of glycan and peptide tags and contributes to the study of glycan fragmentations through LC-MS analysis. Ray is a volunteer educator for the Office of Social Justice and Diversity and is a mentor for the STEM Network.
Rose Mery Bakestani
Rose is a senior undergraduate student who will be graduating with a BS in Biochemistry in Spring 2022. In the lab, Rose uses computational chemistry to calculate bioconjugate fragmentation mechanisms with the lowest possible energy pathways.
Teuta Hida
Teuta is a junior undergraduate student who will be graduating in the Spring of 2023 with a BS in Biochemistry and a minor in Psychology. What drew Teuta to Dr. Gao’s lab was the focus of studying glycans for future potential as early disease biomarkers. When Teuta is not around in the lab, she can be found contributing to the Chemistry Club or continuing to prove to everyone how much of a bookworm she is.
Wilton Gilles
Nathalia Letrari
Alumni
- Nikunj Desai (2014)
- Jungeun Lee (2014)
- Eric Joyce (2015)
- Nathaniel Adomako (2016)
- Trang Do (2016)
- Rosar Najar (2016)
- Jose Acosta (2016)
- Tara Otegui (2017)
- Kimberly Fabijanczuk (2017)
- Kaylee Gaspar (2017)
- Kimberly Calix (2018)
- Edgar Manriquez (2018)
- Maeve Byrne (2020)
Publications
- 2021
- Chunfen Jin, Erlu Feng, Xin Ma, Weijuan Tang, Huaming Sheng, Ashley Wittrig, Jinshan Gao, Hilkka I Kenttämaa, Reactivity of para-benzynes in solution and in the gas phase, Tetrahedron Lett. 2021, 74, 153161.
https://doi.org/10.1016/j.tetlet.2021.153161 - Xin Ma, Erlu Feng, Hanning Jiang, Victoria M Boulos, Jinshan Gao, John J Nash, Hilkka I Kenttämaa*, Protonated Ground-State Singlet meta-Pyridynes React from an Excited Triplet State, J. Org. Chem. 2021, 86, 3249-3260.
https://doi.org/10.1021/acs.joc.0c02594 - Huaming Sheng, Weijuan Tang, Jinshan Gao, James Riedeman, Matthew Hurt, Linan Yang, Hilkka Kenttämaa*, Characterization of Ionized Lignin Model Compounds with α-O-4 Linkages by Positive and Negative Ion Mode ESI/Tandem Mass Spectrometry Based on CAD, Rapid Commun. Mass Spectrom. 2021, 35, e9057.
https://doi.org/10.1002/rcm.9057 - Lei Zheng, Huan Feng, Yueqiang Liu, Jinshan Gao, Dibyendu Sarkar, Yang Deng, Chemically Enhanced Primary Treatment of Municipal Wastewater with Ferrate(VI), Water Environment Research 2021, 93, 817-825
https://doi.org/10.1002/wer.1473 - 2020
- Rayan Murtada, Kimberly C Fabijanczuk, Kaylee Gaspar, Xueming Dong, Kawthar Z Alzarieni, Kimberly Calix, Edgar Manriquez, Rose Mery Bakestani, Hilkka I Kenttämaa, Jinshan Gao*, Free Radical Mediated Glycan Isomer Differentiation. Ana. Chem. 2020, 92, 13794–13802.
https://doi.org/10.1021/acs.analchem.0c02213 - 2019
- Kimberly Fabijanczuk, Kaylee Gaspar, Nikunj Desai, Jungeun Lee, Daniel A Thomas, Jesse Lee Beauchamp*, Jinshan Gao*, Resin and Magnetic Nanoparticle-Based Free Radical Probes for Glycan Capture, Isolation, and Structural Characterization, Ana. Chem. 2019, 91, 15387-15396.
https://doi.org/10.1021/acs.analchem.9b01303 - Kaylee Gaspar, Kimberly Fabijanczuk, Tara Otegui, Jose Acosta, Jinshan Gao*, Development of Novel Free Radical Initiated Peptide Sequencing Reagent: Application to Identification and Characterization of Peptides and Proteins by Mass Spectrometry, J. Am. Soc. Mass Spectrom. 2019, 30, 548-556.
https://doi.org/10.1007/s13361-018-2114-8 - 2018
- Jinshan Gao, Bartłomiej J. Jankiewicz, Huaming Sheng, Lindsey Kirkpatrick, Xin Ma, John J. Nash*, Hilkka I. Kenttämaa*, Substituent Effects on the Reactivity of the 2,4,6-Tridehydropyridinium Cation, an Aromatic σ,σ,σ-Triradical, Eur. J. Org. Chem., 2018, 46, 6582-6589.
http://dx.doi.org/10.1002/ejoc.201801249 - Huaming Sheng, Xin Ma, Haoran Lei, Jacob Milton, Weijuan Tang, Chunfen Jin, Jinshan Gao, Ashley M. Wittrig, Enada F. Archibold, John J. Nash, Hilkka I. Kenttämaa*, Polar Effects Control the Gas-Phase Reactivity of para-Benzyne Analogs, ChemPhysChem, 2018, 19, 2839-2842.
https://dx.doi.org/10.1002/cphc.201800646 - Yang Tang, Yi Pu, Jinshan Gao, Pengyu Hong, Catherine E. Costello, Cheng Lin*, De Novo Glycan Sequencing by Electronic Excitation Dissociation and Fixed-Charge Derivatization, Ana. Chem., 2018, 90, 3793–3801.
http://dx.doi.org/10.1021/acs.analchem.7b04077 - 2017
- Huaming Sheng, Weijuan Tang, Jinshan Gao, James S. Riedeman, Guannan Li, Tiffany M. Jarrell, Matthew R. Hurt, Linan Yang, Priya Murria, Xin Ma, John J. Nash*, and Hilkka I. Kenttämaa*, (−)ESI/CAD MSn Procedure for Sequencing Lignin Oligomers Based on a Study of Synthetic Model Compounds with β-O-4 and 5-5 Linkages, Ana. Chem., 2017, 89, 13089-13096.
http://dx.doi.org/10.1021/acs.analchem.7b01911 - 2016
- Nikunj Desai, Daniel A. Thomas, Jungeun Lee, Jinshan Gao* and J. L. Beauchamp*, Eradicating Mass Spectrometric Glycan Rearrangement by Utilizing Free Radicals, Chemical Science (Royal Society of Chemistry Flagship Journal), 2016, 7, 5390-5397.
http://dx.doi.org/10.1039/c6sc01371f - Emil Tykesson, Yang Mao, Marco Maccarana, Yi Pu, Jinshan Gao, Cheng Lin, Joseph Zaia, Gunilla Westergren-Thorsson, Ulf Ellervik, Lars Malmström and Anders Malmström, Deciphering the mode of action of the processive polysaccharide modifying enzyme dermatan sulfate epimerase 1 by hydrogen–deuterium exchange mass spectrometry, Chemical Science (Royal Society of Chemistry Flagship Journal), 2016, 7, 1447-1456.
http://dx.doi.org/10.1039/c5sc03798k - 2015
- Chang Ho Sohn, Jinshan Gao, Daniel A Thomas, Tae-Young Kim, William A. Goddard, Jesse Beauchamp,* Mechanisms and Energetics of Free Radical Initiated Disulfide Bond Cleavage in Model Peptides and Insulin by Mass Spectrometry, Chemical Science (Royal Society of Chemistry Flagship Journal), 2015, 6, 4550-4560.
- http://dx.doi.org/10.1039/c5sc01305d
- John C Degenstein, Priya Murria, Mckay Easton, Huaming Sheng, Matt Hurt, Alex R Dow, Jinshan Gao, John Joseph Nash, Rakesh Agrawal, W Nicholas Delgass, Fabio H Ribeiro, Hilkka I. Kenttamaa,* Fast Pyrolysis of 13C-Labeled Cellobioses: Gaining Insights Into the Mechanisms of Fast Pyrolysis of Carbohydrates, J. Org. Chem., 2015, 80, 1909–1914.
http://dx.doi.org/10.1021/jo5025255 - 2014
- Jinshan Gao, Bartłomiej J. Jankiewicz, Jennifer Reece, Huaming Sheng, Christopher J. Cramer, John J. Nash,* Hilkka I. Kenttämaa,* On the Factors That Control the Reactivity of meta–Benzynes, Chemical Science (Royal Society of Chemistry Flagship Journal), 2014, 5, 2205-2215.
http://dx.doi.org/10.1039/c4sc00194j - Daniel A. Thomas, Chang Ho Sohn, Jinshan Gao, and J. L. Beauchamp,* Hydrogen Bonding Constraints Lead to Unusual Reactions of Free Radicals with Serine and Threonine Residues in Peptides, J. Phys. Chem. A. 2014, 118, 8380–8392.
http://dx.doi.org/10.1021/jp501367w
Gallery
Click on an image below to enlarge photo.